2024 ACVIM Consensus Statement on ITP
Breaking down the evidence and diagnostic guidelines

Dear Readers,
Today we’ll be discussing the recently published American College of Veterinary Internal Medicine (ACVIM) consensus statement on the diagnosis of immune thrombocytopenia in dogs and cats. This article is going to be one part continuing medical education, one part evaluating the research literature, and one part meta-commentary on evidence-based medicine.
Immune-mediated thrombocytopenia, often abbreviated ITP or IMT, is a deceptively simple condition: The immune-system starts attacking platelets, the little cellular fragments that circulate in your blood to plug leaks in vessels and kick-start clotting. Most patients with ITP have few—if any—platelets and can bleed from that deficiency. The first-line treatment approach relies on suppressing the immune system (often with drugs like steroids). However, behind these basic principles lies a lot of complexity and nuance, and ITP lacks a single definitive diagnostic test.
These consensus statements on various topics are published annually in the Journal of Veterinary Internal Medicine (JVIM) and aim to review the evidence for diagnosis and management of a variety of conditions. These documents are usually very long and technical (this one is 24 pages not including supplemental materials), so I thought it would be useful to publish a shorter and more digestible synopsis of their work.
In this post, I’ll walk through the methodology of the consensus statement on ITP/IMT, the key panel recommendations, and some constructive criticism. Just as an FYI, the figures in this post make it run long and the article may get truncated by your email provider, so you might want to visit the article on the website if that happens.
—Eric
Methodology
This consensus statement was written by over two dozen veterinarians and overseen by the ACVIM Board of Regents. The contributors were an international cohort (primarily in academia) that included experts in clinical pathology, emergency / critical care, hemostasis, and internal medicine. I was not personally involved in these guidelines, although this is a small field, and I know many of the authors. One of the co-first authors was a professor of mine in vet school, two were from Auburn (including my residency mentor and PhD committee member), and several others are friends and colleagues.
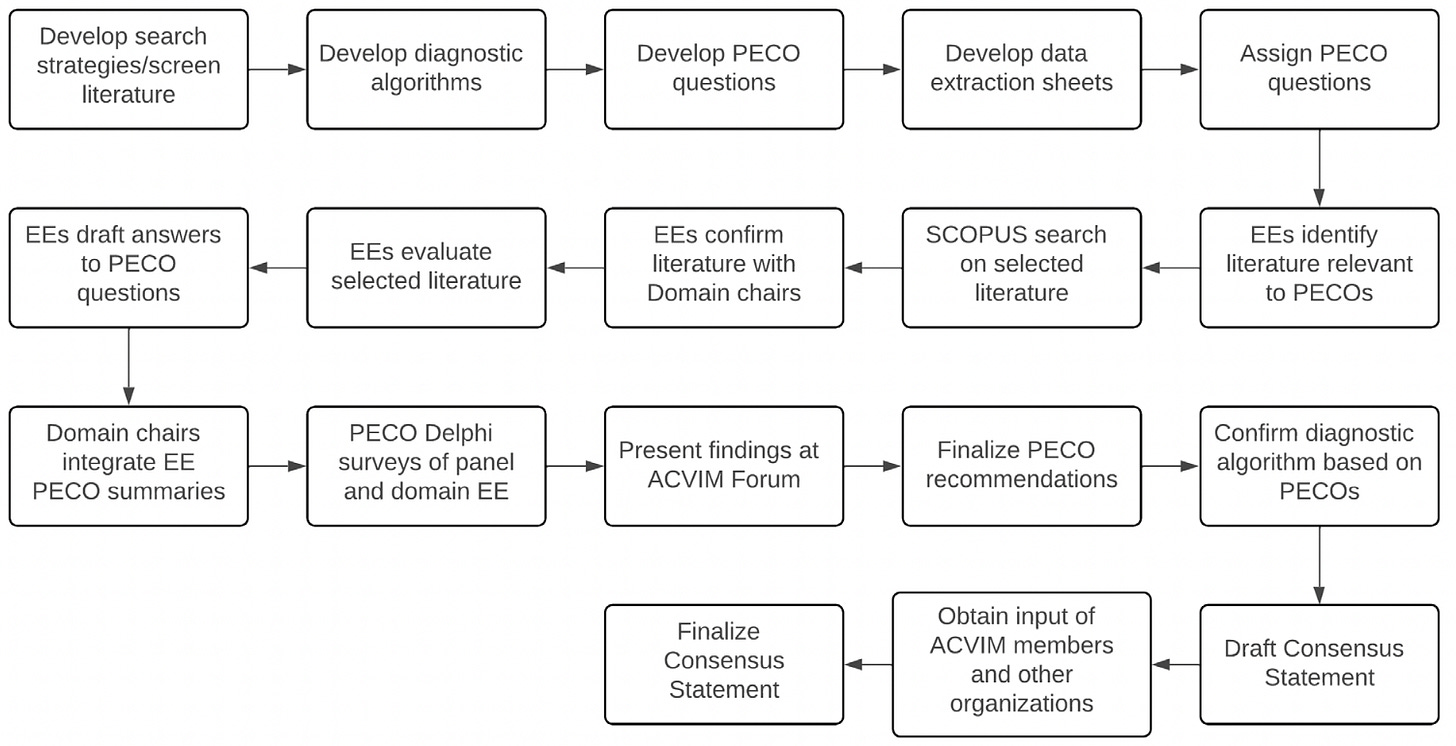
The process for sifting through the research literature, creating questions to evaluate, and comparing published data to expert opinion was quite rigorous, as outlined in this figure from the document:
The evidence was evaluated using structured questions derived from a pool of 287 articles. Evidence evaluators used panel-designed templates and data extraction tools to summarize evidence. Diagnostic algorithms for ITP in dogs and cats were developed and refined through multiple rounds of Delphi review. The final algorithms were integrated after reaching consensus among panel members and professional organizations.
Diagnostic algorithms
There is no way around it: the algorithms produced by this first consensus statement are really complicated. The panel did an admirable job trying to grapple with the complexity of ITP and corner-cases that can result in misdiagnosis, although if I’m being honest, I’m not sure these flow charts are that practical for busy vets seeing 40+ cases a day!
I have copied the diagrams below, but will first provide my high level summary:
VERIFY thrombocytopenia with manual blood smear review !!!
Factors like platelet clumping & large platelets falsely ⬇️ automated [PLT]
This is especially important in cats due to very reactive platelets and lower frequency of true thrombocytopenia
Many pre-analytical factors, like a bad blood draw or inappropriate sample handling can impact platelet count → repeat the test if this is suspected
They recommend an initial platelet cut-off of 100,000/uL based on human medicine, followed by a second cut-off of 50,000 IF there is major blood loss
Make sure to consider congenital macrothrombocytopenia in relevant breeds
Rule out causes of non-immune thrombocytopenia like coagulopathy, sepsis, toxins, and various kinds of bone marrow disease
For patients with probable ITP, it is worth screening for underlying triggers (see section on Co-morbidities below)
Much of the bottom of the algorithm focuses on platelet/megakaryocyte antibody testing to support an immune-mediated component, although as we’ll talk about later, the literature review portion de-emphasized using those tests for initial diagnosis, and you can diagnose ITP without them.
Dogs:
Cats:
Literature review: Diagnosis
The literature review was divided into two domains: Diagnosis and Comorbidity. For Diagnosis, the authors used structured Population Exposure/Evaluation Comparison Outcome (PECO) questions to guide their review of studies. They rated both the level of evidence (low, moderate, high) and the strength of their recommendation (weak, moderate, strong). Since the vast majority of the veterinary literature is comprised of retrospective and observational studies and case reports, the degree of evidence in all cases was weak to moderate. I have stratified their recommendations by those two parameters.
✅ Coagulation testing is critical in working up thrombocytopenia (4.5.1)
For cats and dogs with thrombocytopenia, multiple studies demonstrate that a high proportion of cases with thrombocytopenia have hemostasis abnormalities. As such, coagulation tests (activated partial thromboplastin time and prothrombin time) are recommended as part of the routine evaluation. Additionally, consider measuring fibrinolysis markers, such as fibrin degradation products (FDP) and D-dimer.
✴️ A platelet count <20,000/uL is supportive of ITP, but *can’t* diagnose it by itself (4.2.1)
Available evidence suggests that dogs with ITP generally have more severe thrombocytopenia than those with non-immune causes, though there is some overlap. Assessing the severity of thrombocytopenia can help differentiate between ITP and non-immune causes. A platelet count below 20,000/μL supports an ITP diagnosis but is not sufficient alone to confirm it.
The panel reached similar conclusions about thrombocytopenic cats, but deemed the evidence lower quality and labeled the strength of recommendation as “weak.”
✴️ Admission platelet count is NOT correlated with prognosis (4.2.2)
There was moderate quality evidence that the admission [PLT] did not correlate to survival or response to treatment. The panel recommended that initial platelet count alone should not be used as a prognostic factor.
✴️ Severity scoring is recommended to aid prognostication (4.5.2)
In contrast, multiple cohort and retrospective studies suggest anatomic site of bleeding, severity scoring (such as the DOGiBAT bleeding assessment tool) and presence of melena are associated with transfusion requirements, duration of hospitalization, and survival.
✴️ CBC and chemistry panels are recommended to aid severity scoring (4.6.1)
Evidence from case reports and case series indicates that abnormalities in biochemistry panels (high BUN) and CBCs (anemia) may be linked to more severe disease, transfusion requirement, and poor prognosis, although results across studies were mixed.
📌 Platelet indices should NOT be relied on for diagnosis (4.1.1)
There is conflicting evidence about mean platelet volume (MPV) changes in dogs with ITP compared to other causes of thrombocytopenia or healthy dogs. Increased reticulated platelets may help differentiate ITP from non-immune thrombocytopenia but not between primary and secondary ITP. There is insufficient evidence to recommend using plateletcrit and immature platelet fraction (IPF) for diagnosing ITP in dogs. Therefore, using plateletcrit, IPF, and MPV as routine tests for primary ITP is not recommended. However, if available, measuring increased reticulated platelets can support a diagnosis of primary or secondary ITP in thrombocytopenic dogs.
📌 Platelet indices do NOT reliably predict treatment response (4.1.2)
In dogs with primary ITP, weak evidence suggests plateletcrit is more sensitive than platelet count for detecting platelet recovery. There is insufficient evidence to determine the utility of MPV, reticulated platelets, or IPF in predicting bleeding severity, hospitalization duration, blood product needs, or platelet count recovery. Thus, MPV, reticulated platelets, and IPF are not recommended as routine prognostic tools. However, they suggest considering serial plateletcrits alongside platelet counts to assess treatment response in dogs with ITP.
📌 Bone marrow evaluation is NOT recommended for routine ITP work-ups (4.3.1 & 4.3.2)
There is insufficient evidence to confirm that bone marrow examination improves the diagnosis of primary ITP in dogs or predicting outcome. Therefore, it is not recommended as a routine diagnostic test for primary ITP. However, bone marrow examination can be considered for characterizing ill-defined cytopenias, with the understanding that no specific bone marrow abnormality is diagnostic for primary ITP.
📌 Platelet/megakaryocyte-associated antibody tests are NOT recommended for diagnosis (4.4.1), but MAY predict relapse (4.4.2)
In thrombocytopenic dogs, positive platelet/megakaryocyte-associated antibody tests suggest an immune component to the thrombocytopenia but are not diagnostic for ITP. Routine measurement of these antibodies is not recommended for diagnosis. However, serial monitoring of these antibodies might help identify disease relapse.
Literature review: Co-morbidities
For the co-morbidity section, the authors did not use PECO questions. Instead, they assessed individual publications concerning possible triggers for secondary ITP using an Integrated Metric of Evidence (IME) score to determine the strength of evidence for each comorbidity. They found that the evidence for a causative association with ITP was the highest for infectious diseases. Evidence to support drug-associated ITP was low, and for other conditions there were insufficient number and/or quality of studies to make a recommendation.
Specific infections that had a high level of evidence for association with ITP included Ehrlichia canis, Leishmania infantum, Rangelia spp., and canine distemper virus. There was intermediate level evidence for Anaplasma spp. (both A. phagocytophilum and A. platys) and low-level evidence for Babesia spp. Other infectious causes were of low-quality evidence and/or lacked studies to apply the diagnostic algorithm and IME rubric.
Based on this section, the authors made the following recommendations for screening dogs with ITP to look for underlying triggers:
For all dogs and cats
Thorough physical exam
History
Including questions about drugs, vaccines, travel, flea/tick/heartworm exposure & prevention
Complete Blood Count (CBC) with pathologist or medical technologist review
Serum biochemistry
Urinalysis
At the discretion of clinician based on case findings:
Imaging
Urine culture
Fecal flotation screen for ova & parasites
Infectious disease testing specifically for animals living in endemic regions:
Dogs
Ehrlichia spp.
Anaplasma spp.
Babesia spp.
Leishmania spp.
Rangelia spp.
Angiostrongylus vasorum
Canine distemper virus (especially if non- or lapsed vaccination)
Cats
Feline Immunodeficiency Virus (FIV)
Feline Leukemia Virus (FeLV)
A. phagocytophilum
Ehrlichia spp.
Babesia felis
Strengths and weaknesses
First, I want to acknowledge the significant effort that went into this consensus statement, involving hundreds of studies and multiple stakeholder consensus meetings. While this review on diagnosing ITP in dogs and cats is imperfect—understandable for any document seeking professional consensus on a complex disease without a gold standard test—it gets the big questions right and offers a valuable framework for current practice and future research. The guidelines emphasize confirming platelet counts with blood smear reviews, considering pre-analytical variables, and ruling out common mimics like breed-associated thrombocytopenia and coagulation problems.
However, the guidelines have several notable weaknesses. The most significant, which the authors acknowledge, is the lack of high-quality prospective studies and randomized clinical trials, a common issue in veterinary consensus statements. A rigorous evidence-based medicine approach can't compensate for the absence or poor quality of underlying research. These knowledge gaps hinder progress in veterinary medicine and are a topic I'll write about in the future.
Additionally, the sheer complexity of the diagnostic algorithms and guidelines limit their real-world utility. In many sections, I had to re-read passages many times to wrap my head around what the authors were trying to communicate, and I specialize in this area! The amount of space dedicated to anti-platelet/megakaryocyte antibody testing in particular strikes me as excessive, given the high false-positive and false-negative rates:
Finally, while I agree with most recommendations, in my opinion some statements are not supported by my experience or research. For example, in the section explaining the diagnostic algorithm the authors state:
“Our diagnostic algorithm begins with a confirmed platelet count of <100,000/μL, consistent with ITP definitions in human medicine, and a relevant threshold for likely ITP diagnoses in animals. Some animals with ITP may have higher platelet counts at diagnosis [my emphasis], but this threshold increases the likelihood of an ITP diagnosis”
In my opinion, for animals with a platelet count >50,000/μL, alternate diagnoses should be considered, and counts >100,000/μL almost certainly exclude primary ITP for first-time diagnoses. Secondary ITP caused by other diseases is more complex because they can cause thrombocytopenia through multiple mechanisms like vasculitis and clotting issues in addition to a hypothetical immune-mediated component, and it is not always possible to disentangle these processes.
To improve these guidelines, future research should address the identified knowledge gaps, especially in areas with weak or absent studies. Simplifying the guidelines could further enhance their usability.
Final thoughts
Overall, this consensus statement provides a comprehensive literature review through structured PECO questions, Delphi review process, and detailed diagnostic algorithms. Input from multiple experts and organizations boosts its credibility. However, the algorithms are overly complex, and the recommendations are limited by issues in the veterinary research literature. Despite this, it’s a strong initial effort that could improve with more research and some simplification.
The bottom-line: Like many immune-mediated diseases, ITP remains poorly understood and lacks a single gold standard test, making it a diagnosis of exclusion. Key steps in the work-up include:
Verifying platelet counts with manual blood smear reviews
Excluding breed-specific conditions and non-immune causes of thrombocytopenia (particularly clotting disorders and bleeding)
Conducting a thorough history, physical exam, and testing for potential triggers of secondary ITP










Thank you so much! That definitely makes what happened a little clearer. The dog did present with those symptoms (107 fever and the rest of it) but the circumstances were a little strange given it was about 60 degrees Fahrenheit that day.
Somewhat technical for a non-veterinarian, but very useful for someone trained!