How AI Accelerates Drug Development
Deep-learning is discovering brand new life-saving medications
Dear Readers,
I’ve written a lot about AI in this newsletter (see here, here, and here, for just a few). Much of my work has focused on how AI may disrupt education and how doctors interact with patients and medical systems. An area I have not focused on as much is the potential for AI to radically accelerate research and development and lead to new breakthrough treatments. This is something AI optimists have been most excited about and we’re finally starting to see this promise materialize. In this edition of All Science, the first portion of the article provides an overview of how AI impacts the drug-development process and is free for everyone, while the full article that goes into specific examples is for paid subscribers.
—Eric
Deep-learning in drug discovery
The pharmaceutical industry is a high risk business—almost 90% of new drug candidates ultimately fail. The process of discovery and clinical trials is arduous, inefficient, and filled with trial-and-error. Any methods to improve this pipeline could be a boon to innovation and reduce costs.
This is where AI comes in: Deep-learning algorithms, a subset of machine learning, are adept at handling large datasets and complex patterns. In drug discovery, AI can rapidly screen tens of thousands of compounds to find subtle clues that identify new drug candidates, a process that could take years or decades for humans to do manually. Many experts believe AI could significantly reduce the time taken to identify molecules with potential therapeutic effects, leading to life-saving medications faster than ever.

AI's ability to predict molecular behavior is revolutionizing drug design. For instance, Google’s DeepMind developed AlphaFold, an AI system that predicts protein folding structures, a key factor in understanding diseases and developing drugs. Accurate predictions of protein structures can drastically reduce the trial-and-error aspect of drug design. AlphaFold recently released their predicted 3-D structures for hundreds of thousands of proteins, including almost all of those in our bodies, solving a long-standing challenge in biochemistry and molecular biology.
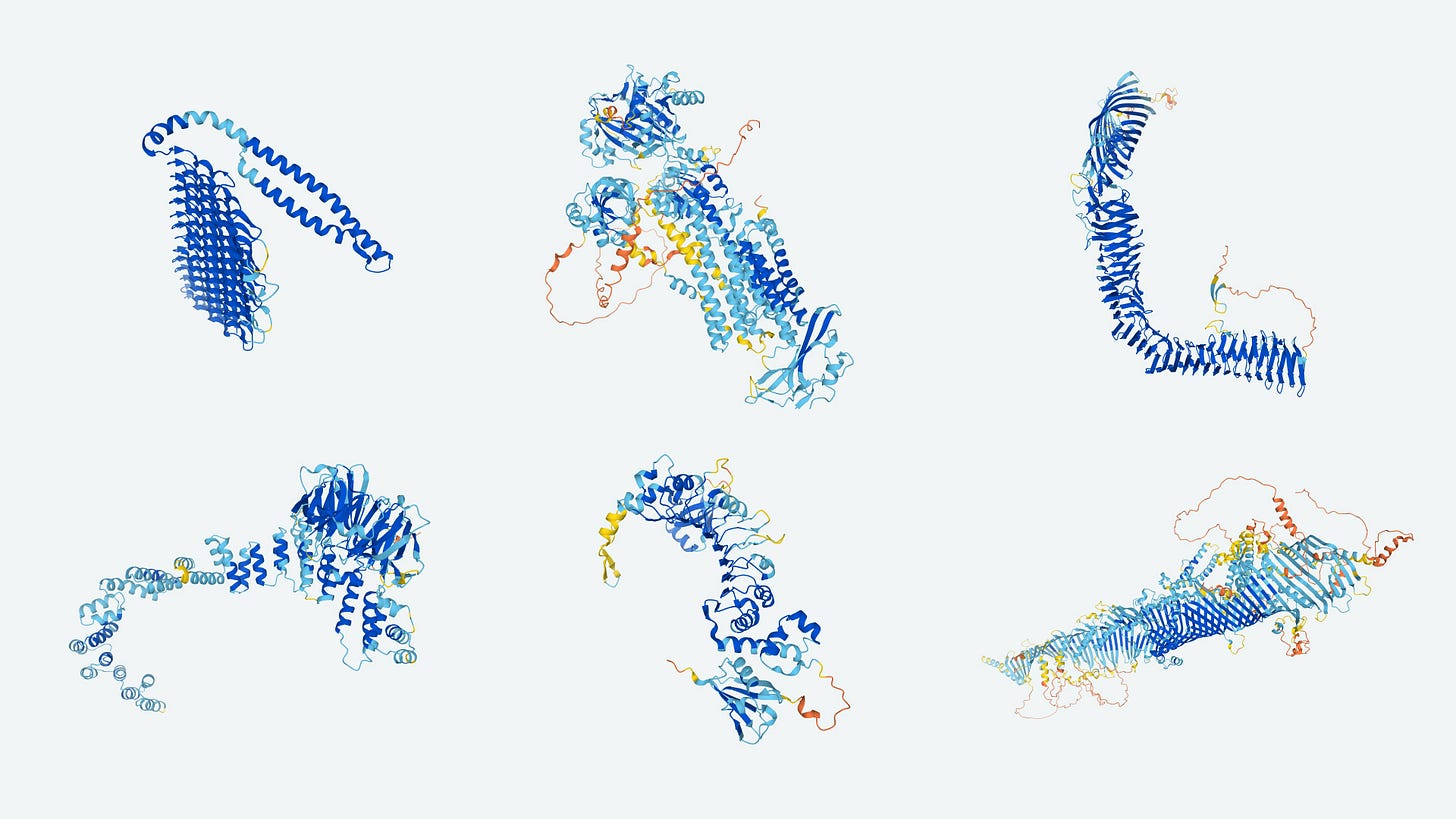
AI may also streamline clinical trials. By analyzing patient data, AI can identify suitable candidates for studies, ensuring faster and more efficient enrollment. Furthermore, AI can monitor trial progress in real-time, allowing for quicker adjustments and decision-making. This has the potential to cut both the time and cost of drug development in half. Similarly, deep-learning AI can also pave the way for personalized medicine: by analyzing genetic data, AI could tailor treatments for patients based on their specific genes that metabolize drugs, potentially increasing their efficacy (this new field is called pharmacogenomics).

Given all of this potential, let’s look at some real world examples of these benefits in action ⤵️
Deep-learning discovers brand new classes of antibiotics for resistant infections
Antibiotic resistance is a growing problem that threatens to reverse progress made against bacterial illnesses:
“The global rise in antibiotic resistance poses a significant threat, diminishing the efficacy of common antibiotics against widespread bacterial infections. The 2022 Global Antimicrobial Resistance and Use Surveillance System (GLASS) report highlights alarming resistance rates among prevalent bacterial pathogens. Median reported rates in 76 countries of 42% for third-generation cephalosporin-resistant E. coli and 35% for methicillin-resistant Staphylococcus aureus are a major concern. For urinary tract infections caused by E. coli, 1 in 5 cases exhibited reduced susceptibility to standard antibiotics like ampicillin, co-trimoxazole, and fluoroquinolones in 2020. This is making it harder to effectively treat common infections.”
Similar treatment resistance is occurring in fungal and viral diseases as well.
That’s why it’s so exciting that researchers at MIT and the Broad Institute discovered novel antibiotics to treat these challenging pathogens through AI. In one study, this research team explored the use of deep learning to identify new antibiotics against Acinetobacter baumannii, a Gram-negative pathogen known for its multidrug resistance (sometimes referred to as a “superbug” by the popular press). The study involved screening about 7,500 molecules and using a neural network trained on this dataset to predict new molecules with potential antibacterial activity against A. baumannii. This approach led to the discovery of abaucin, an antibacterial compound with narrow-spectrum activity against A. baumannii. Further investigations revealed that abaucin disrupts lipoprotein trafficking through a mechanism involving the protein LolE. Additionally, abaucin was effective in a mouse wound infection model, suggesting its potential as a novel treatment for infections caused by A. baumannii.
Their work expanded dramatically in a follow-up study. They used an AI system to screen millions of chemical compounds, leading to the identification of compounds effective against Methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus. Their deep learning model, trained on a large dataset, efficiently predicted compounds that not only showed promise as antibiotics but also had low toxicity to human cells. Even more exciting, they discovered that their AI models identified an entirely new class of antibiotics! These findings were validated in vivo through mouse experiments, demonstrating their effectiveness against skin and thigh infections caused by MRSA. This breakthrough is significant as it introduces a new class of antibiotics, one of the few discovered in the past 60 years, and shows the potential of AI in drug discovery.
Machine learning genomics can identify personalized cancer therapies
Another recent study has made significant strides in understanding how genetic alterations in cancer affect drug sensitivity and resistance through AI. Researchers analyzed over 11,000 tumors from various tissue types, combining data on genetic mutations, changes in DNA copy number, methylation patterns, and gene activity. They then matched these findings with how 1,001 cancer cell lines responded to 265 different drugs.
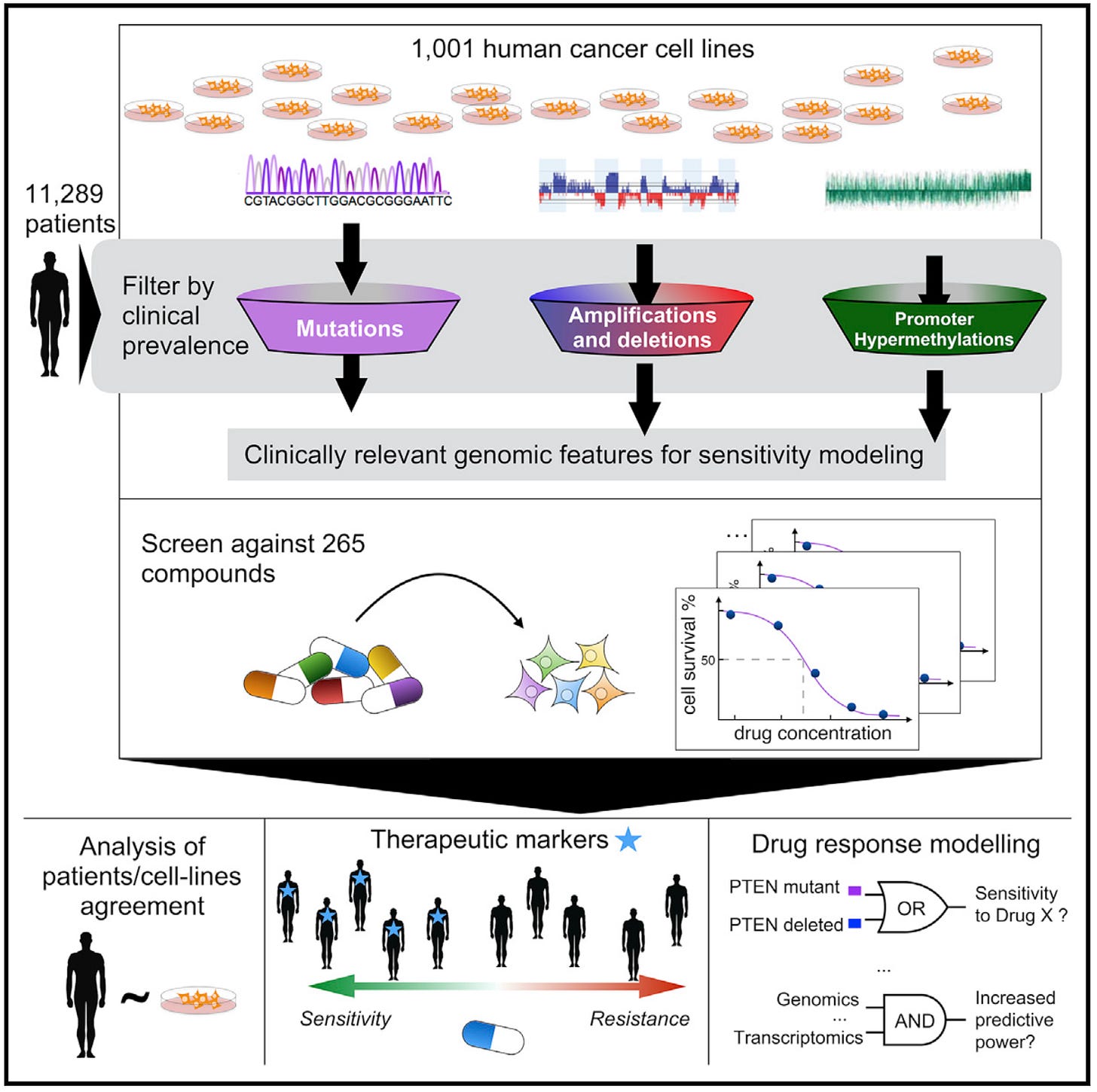
The researchers used machine learning to examine large-scale cancer genome databases and create a detailed map of cancer-related genetic changes. This analysis revealed that most tumors have only a few key mutations that drive their growth. The study then explored how these genetic factors affect a tumor's sensitivity to various drugs, producing a comprehensive dataset of drug responses.
This research highlights how big data and advanced computational methods can be used to better understand and treat cancer. By linking specific genetic changes in tumors to drug responses, the study paves the way for more personalized cancer treatments. It's a prime example of precision medicine, where treatments are tailored to the unique genetic profile of each patient's tumor, potentially leading to more effective and targeted therapies.
Generative AI is designing molecules for drug trials
The previous examples have highlighted how AI can be used to improve screening for promising compounds or cellular targets. However, some companies are now exploring using AI to design the very chemical structure of their drugs! Numerous drugs designed in part or in total by AI are already entering clinical trials:

One such drug, INS018_055 by Insilico Medicine, marks a significant advancement in AI-assisted drug discovery for treating Idiopathic Pulmonary Fibrosis (IPF), a serious lung condition. Here's how it was developed: Insilico used two AI platforms, PandaOmics for finding a new target called "Target X" in the fight against lung fibrosis, and Chemistry42 for designing the actual molecules. Out of 79 molecules created by the AI, the 55th one, named INS018_055, showed potential in improving lung fibrosis and was safe in mouse models. It has been tested in early-stage clinical trials with 78 healthy volunteers in New Zealand and China, showing good safety and tolerability. Recognizing its potential, the FDA granted it Orphan Drug Designation in February 2023, a special status that supports drugs developed for rare diseases.
This drug is a big deal in the world of medicine for a few reasons. First, it's one of the first examples where AI was used not just to pick a target for treatment but also to design the drug. For patients with IPF, a disease with limited treatment options, INS018_055 offers new hope. The drug is now moving into more advanced trials, where its effectiveness and safety will be tested in IPF patients. This development demonstrates the power of AI in revolutionizing drug discovery and providing new solutions for challenging medical conditions.
Towards the future
Despite its potential, the integration of AI in drug development faces challenges, including data quality and ethical concerns. However, as technology advances and regulatory frameworks evolve, the role of AI in drug development is set to expand further, offering exciting prospects for the future of healthcare. The FDA is already discussing how to integrate and evaluate AI into the traditional pharmaceutical approval process, as are similar organizations in Europe.
Deep-learning AI is likely to be a game-changer for human—and hopefully veterinary—drug development. Its ability to analyze complex biological data, enhance drug design, streamline clinical trials, and personalize medicine is already transforming the field, promising to bring new, effective treatments to patients more quickly and efficiently. As we continue to harness the power of AI, the future of drug development looks brighter than ever.






