As anyone who has needed an x-ray, ultrasound, or CT scan in recent memory knows, radiology has been all-digital for decades now—gone are the days of actual film developed in a dark room. Likewise, biopsy histopathology has gone digital, with veterinary laboratories leading the way ahead of human laboratories, which didn’t start to digitize in earnest until the FDA cleared the first whole slide imaging (WSI) scanner for diagnostic use in 2017. Today, nearly all veterinary anatomic pathologists in North America are reading virtual slides on their computers from home, while in human medicine the transition has lagged due to high costs, regulatory barriers and slow change management.
More recently, veterinary cytology has started going digital, with disruptive start-ups such as Lacuna Diagnostics (where I was a co-founder) and Scopio Labs entering the scene around 2017-20181. Digital cytology has created bigger waves than digital histopathology because improving technology and falling costs have allowed placing scanners into individual vet clinics for STAT point-of-care (POC) results in 1-2 hours, often available 24/7.
In this article, I will talk about the pros and cons of this emerging scanning technology with a focus on published studies and some examples from my own experiences with different modalities.
Why Go Digital?
Compared to digital radiology—where the new technology eliminates a lot of the old cumbersome workflow and exposure to toxic chemicals—digital pathology only adds costs and steps, as biopsy slides still need to be prepared and stained the traditional way prior to scanning:
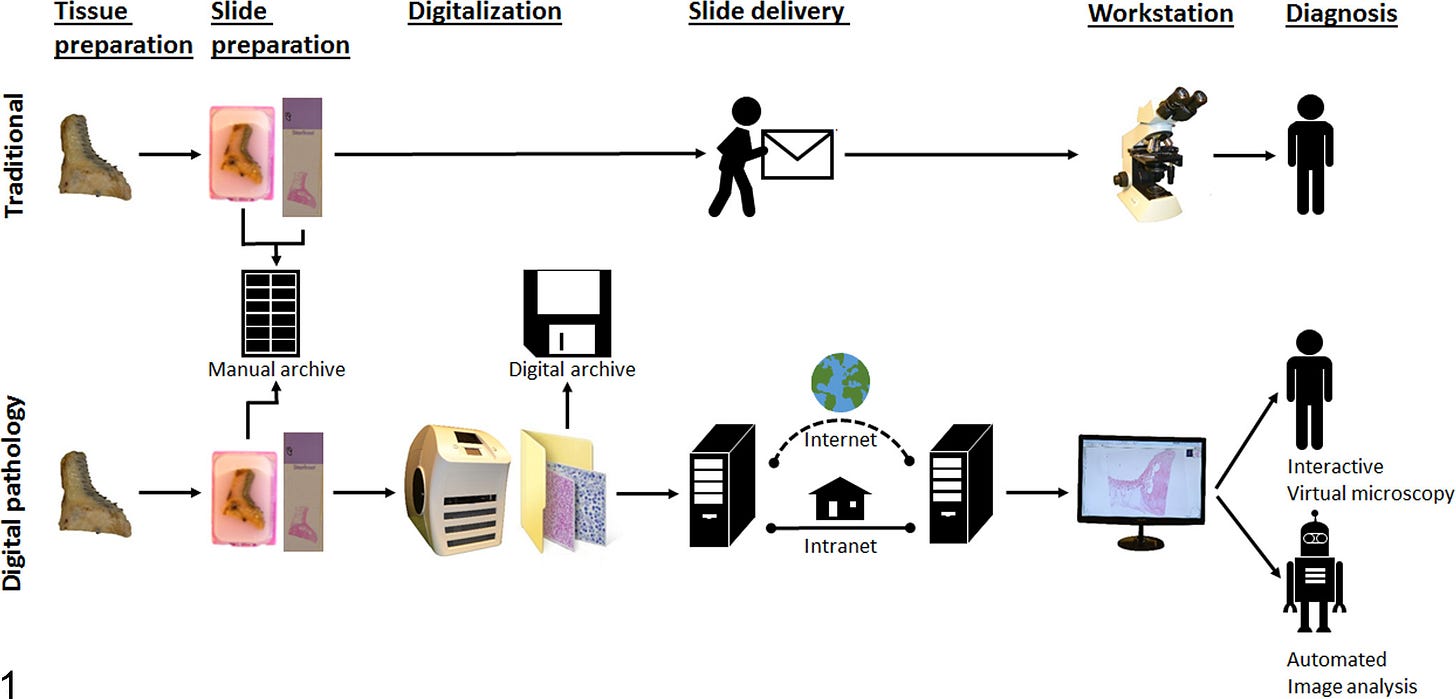
As you can see, the new process certainly has some drawbacks. For digital cytology specifically, these can include:
Resolution concerns
Majority of scanners on market cannot scan at 50-100x oil magnification
Can make it harder to see small structures like bacteria, granules, etc
Expensive scanning hardware (often six-figures per scanner)
More complicated lab workflow
IT issues → transfer and storage of large files over internet
Slow scan times of 5-10+ minutes per slide for cytology
Adding “z-stacking” (multiple layers) can exponentially increase scan time
Have to read a less traditional stain type for POC cases (Diff-Quik)
CHANGE MANAGEMENT → who moved my 🧀 ?!?!?!?
Given the added complexity and costs, why would anyone voluntarily adopt digital pathology, especially cytology? In fact, there are a number of really powerful advantages, such as…
Allows remote reading!
Flexibility & quality of life for consultants
Resiliency → Labs with personnel shortages can re-route cases
This was particularly useful for COVID-19 disruptions
Easy to get second opinion case consults between colleagues worldwide
Can improve confidence and quality of diagnosis
Point of care = rapid diagnosis
Minutes or hours can count for critical conditions that need surgery, antibiotics, transfusions, chemotherapy, etc
Permanent archive of slide materials
Allows image analysis & AI applications
Validating Veterinary Digital Pathology
Despite digitizing faster, veterinary pathology has done unfortunately less research or validation of their offerings than their far more cautious and regulated human medical counterparts.
WSI Histopathology
To my knowledge, the first validation study of any sort for histopathology of skin tumors was published by Bertram et al in Veterinary Pathology in 2018 (an academic group of collaborators in Germany and Switzerland). This was notably several years after the majority of commercial diagnostic labs in North America had already gone digital.
The study design was blinded and randomized evaluation of 80 histologic skin sections from 17 tumor types by by 6 pathologists with different levels of experience at 4 time points (cases read twice on glass and twice on digital). Pathologists were not given any history or clinical information beyond species (i.e. dog, cat). They were asked to provide a freeform diagnosis that was not restricted to a specific list. Their intra-observer variation between time points (how often they disagreed with themselves) was compared between the traditional and digital modalities to see if they were significantly different.
While they found that the new technology was roughly interchangeable, it was not perfect:
“Comparison of both methods found digital microscopy to be noninferior for differentiating individual tumor types within the category epithelial and mesenchymal tumors, but diagnostic concordance was slightly lower for differentiating individual round cell tumor types by digital microscopy. In addition, digital microscopy was associated with significantly shorter diagnostic time, but diagnostic confidence was lower and technical quality was considered inferior for whole-slide images compared with glass slides.”
One of the main limitations of that study was the fact that they only evaluated tumors, and not a wider variety of lesions such inflammatory or immune-mediated diseases, infectious agents, etc. Some of these, such as diseases with rare bacteria, may be just as difficult to identify as some of the round cell tumors, although the addition of special histochemical stains could mitigate some of this risk.
WSI Digital Cytology
The first validation study of WSI digital cytology was carried out by a different academic research group in Italy, Bonsembiante et al in Veterinary Pathology 2019. The cytology slides were scanned at 400x with 7 different “z-plane” layers captured, so pathologists could browse up and down in 3D. Their study design was similar to the Bertram et al paper above, although they used three pathologists with varying experience, a total of 60 cases split across 13 categories (most were tumors, but about a quarter of cases were inflammatory or benign), and participants classified diagnoses by choosing from a pick-list of possible options.
They found:
“No significant differences were noted between digital and glass slides in regard to the number of cases correctly diagnosed, or the sensitivity, specificity, or diagnostic accuracy, irrespective of the observers’ expertise. The agreements between the digital slides and the gold-standard examinations were moderate to substantial, while the agreements between the glass slides and the gold-standard examinations were substantial for all 3 observers. The intraobserver agreements between digital and glass slides were substantial to almost perfect.”
The key table of performance by diagnosis can be found below:
These are very strong results that support the viability of WSI digital cytology, although I want to point out two things that limit its real world external validity. First, choosing a structured diagnosis from a list is going to innately limit diagnostic variation and make the results look better compared to the other studies I discuss. Second, scanning 7 z-planes on an entire slide will improve the diagnostic quality compared to evaluating a single plane, but it takes an incredible amount of time—can be several hours!—so it is often not done in the real world.
Region of Interest (ROI) Digital Cytology
The previous studies have all focused on whole slide imaging. But some scanners capture a subset, or region of interest (ROI), of the entire slide to improve scanning speed, reduce file size and post-imaging software processing, or other reasons. In addition, ROI scanning hardware is usually more cost-effective than more advanced WSI scanners.
To evaluate what impact scanning a portion of slides had, Blanchet et al published their study of ROI digital cytology in Veterinary Pathology in 2019 (Disclosure: I was a study pathologist and co-author, and worked at the company that designed the project). They scanned different ROI on the slides on a Nikon E200 microscope with FLIR Blackfly camera at 10x, 40x, and 60x.
The design in this study was similar to those above, including 60 slides of varying diagnoses. There was more balance among lesion types in this study, with 31/60 cancer cases, 14 cases of inflammatory +/- infectious disease, and 15 cases of benign proliferations. Two blinded pathologists read randomized cases by ROI-digital cytology or glass, while two unblinded pathologists selected the cases and scored the unstructured pathology reports as fully concordant, partially concordant, or discordant.
Diagnostic concordance between the pathologists and modalities was overall robust, particularly for malignant neoplasia compared to benign proliferations and infectious/inflammatory disease:
In the Discussion, the authors explain their thoughts on the discordant cases:
“…discordance was likely related to selection bias introduced by the scanning technician selecting nonrepresentative slide areas for ROI-DM capture, which was an intrinsic risk of a nonpathologist performing the scanning. Several examples of this issue occurred in this study, such as when both pathologists found the ROI-DM scan in case No. 33 nondiagnostic because it only contained blood. Similarly, a slide of a benign adnexal tumor in case No. 38 resulted in discordance because the ROI-DM scan overemphasized an area with mesenchymal cell and extracellular matrix components relative to the basal cell component.”
This highlights the importance of full WSI scans, or at least making sure parties are aware of the risks of scanning subsection ROIs, and take steps to mitigate this issue.
Static Telecytology (Still Images)
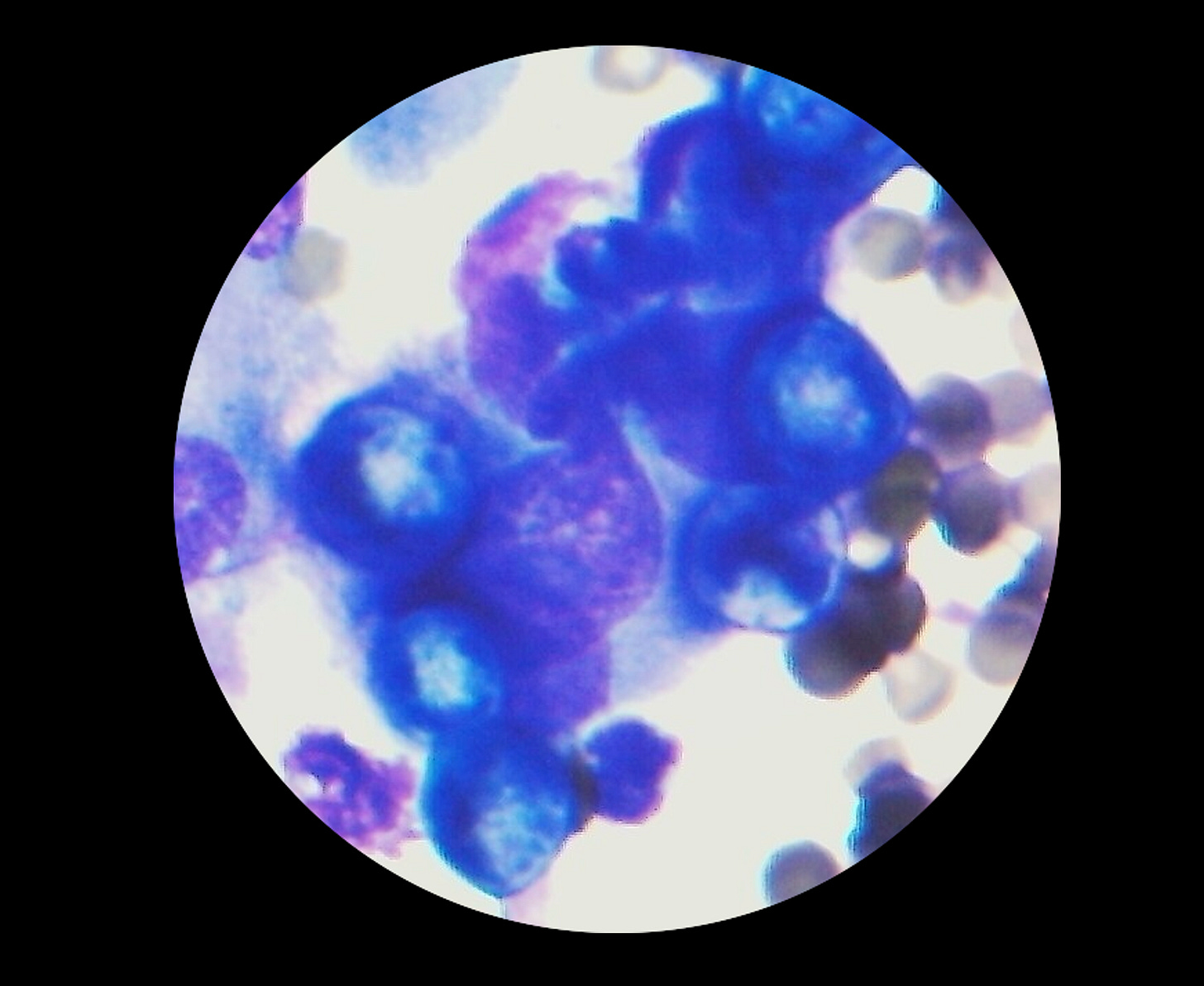
The above photo is from a real consult I had several years ago. I was able to quickly diagnose a cat with the fungal infection Blastomyces dermatidis from cell phone pictures of a lung aspirate. The cat was able to get treatment within hours, which otherwise would have taken days or up to a week at a traditional lab. This is a great example of the potential value of this type of consult.
One of the secondary effects of a proliferation of smartphones is everyone has a high-quality camera in their pockets at all times. This has enabled veterinarians to get telemedicine consultation from pictures of lesions, radiographs, labwork, and even microscope findings. Sometimes these are informally posted on social media or sent via text for second opinions. There are also several companies that offer fee-for-service static telecytology consultations. This trend is not without controversy, as some clinical pathologists think still images aren’t diagnostic quality.
It is true that taking a subset of images creates the risk of misinterpretation based on submitting non-representative areas of the slide(s) similar to the study by Blanchet et al above. If anything, this risk is greater for photos compared to a larger ROI scan. Sometimes the samples prepared in clinic are not up to par quality-wise for one or more reasons, ranging from staining to equipment settings. Some phones and attached microscope cameras also vary in resolution.
So what does the research literature have to say about this modality? Two small studies have looked at the question whether still photographs evaluated remotely can be diagnostic.
The first study to evaluate still image diagnosis was published by the Italian academic group Maiolino et al in Veterinary Clinical Pathology in 2006. They sent 130 images from 20 cases with a consensus light microscopy diagnosis to a blinded remote consultant. The table of results from the paper can be found below:
The one case of disagreement was due to a low cellularity sample and poor image quality where the pathologist could not provide a diagnosis. The two cases of minor disagreement were due to very subtle differences in classification that I don’t even practice myself (I usually bottomline those lesions as perianal tumors, and say that most are adenomas, but it can be difficult or impossible to differentiate those from epitheliomas or carcinomas).
The authors concluded:
“Our results emphasize the numerous advantages of static telepathology by Internet e-mail, particularly:
low cost: at present to make a telediagnosis it is sufficient to have a camera attached to a microscope, which in turn is attached to the Internet;
speed of preparation: the time taken to prepare a virtual slide is approximately 30 minutes;
rapid diagnosis: diagnosis takes from a few minutes to 1– 2 days. This is much less time than is required to receive a report by sending the slides to a consultant through courier or postal mail. Digital images are easier and faster to examine, as the consultant reviews only a small number of selected images rather than screening manually the whole slide with a microscope;
diagnostic accuracy: accuracy is very close to that with conventional glass slide light microscopy.
In conclusion, the results of this study show that telediagnosis through Internet e-mail provides acceptable efficacy and a faster turnaround time than post and can be applied in veterinary diagnostic cytology.”
Another study by an academic group in the US asked a slightly different version of this question. Brooker et al (Veterinary Clinical Pathology 2019) wanted to know if there was an impact on diagnostic accuracy for the level of experience of the person taking cytology photos or number of photos taken. Not surprisingly, they found that people with more cytology training taking photos improved accuracy, as did submitting more images than fewer:
“Clinical agreement between the image and glass slide diagnoses was significantly higher when images were taken by an experienced rather than inexperienced cytologist after the evaluation of two (P = .007) and five images (P = .008). The telecytology diagnoses agreed with the gold standard diagnoses more frequently after evaluation of five images rather than two when images were captured by both the experienced (P < .001) and inexperienced cytologist (P < .001).”
Current and Future Recommendations
This article summarizes the main five studies I’m aware of evaluating digital pathology (particularly cytology) in veterinary medicine. This is a tiny drop in the bucket compared to the human medical literature, and I sincerely hope we can ramp up the amount of research in this area so we have the data to make the best decisions for this new technology.
I’m not here to say one lab’s scanning platform is better than another, and this is not meant to be an endorsement of any specific company. For one thing, the hardware in this space keeps rapidly changing, and some of the details are proprietary, so it is difficult to directly compare the various options, and any conclusions are likely to change in the next year or two. Second, from my experience with a half dozen different scanners, there is no current gold standard technology that balances all of the key considerations in terms of price, throughput, optical resolution, ease of IT implementation, etc.
Unfortunately, there are currently no published professional guidelines on the validation or use of digital cytology in veterinary medicine, although they will likely be coming in the near future. In the mean time, the onus is on labs to follow best practices for validation on their own, and I would suggest starting with the College of American Pathologists (CAP) guidelines on WSI validation from human medicine.
For individual vets and clinics, I would recommend asking questions of your lab, such as these:
What scanner are you using? How did you validate it?
What are the limitations of your platform? Can it see small structures like bacteria or fine granules?
Can you show me either peer-reviewed publications or internal study data?
Do I have the option to request z-stack scanning or a review of the full glass slide in certain cases to make sure nothing is missed?
For those considering the static telepathology model, there are additional considerations for optimal outcomes. As the Brooker et al study above suggests, it is preferable to have the person with the most cytology experience possible taking photos, and at least 5 images are suggested (more is better)—capturing multiple unique fields at low to high magnification is best practice. To optimize the actual photos you take, the clinical pathology lab at Texas A&M recommends the following:
“General tips for taking photographs through the microscope: 1) Clean microscope eyepieces and phone camera; 2) ensure proper microscope settings; hold phone sideways, lean both hands/fingers against eyepieces to stabilize the phone; move phone camera closer until you see contents of microscope slide; 3) make slight movements to center the light in the image; 4) make slight movements to widen the circle/move closer; eyepiece should not be visible in your picture; 5) tap screen to focus or allow phone to autofocus, as applicable; 6) crop and zoom pictures after capturing (optional). [Credit: Dr. Julie Piccione].”
There are also commercially available digital microscope cameras as well as adapters specifically designed to mount smartphones to microscope eyepieces that can make it easier to take higher quality photos.
I hope this discussion of studies and tips helps you understand the current digital cytology landscape a little better. This is a fast moving area of technological change in veterinary medicine, and I’m sure this won’t be the last time I write about it!
What has your experience with digital pathology been? Do you have any questions or concerns you want to discuss? Drop a line in the comments below 👇
Note that neither Lacuna Diagnostics nor Scopio Labs exists in their original forms anymore. Lacuna was acquired by Heska (which was recently snapped up by VCA-Antech), and Scopio Labs exited the veterinary market to focus on their hematology products for the much larger human medical market.